Give the formula of the following compounds:
(i) Magnesium bicarbonate.
(ii) Cupric oxide.
(iii) Ferric oxide.
(iv) Ammonium hydroxide.
(v) Calcium carbonate.
(vi) Potassium carbonate.
(i) Magnesium bicarbonate:
Valency of magnesium = 2
Valency of carbonate = 2
By applying criss-cross method:
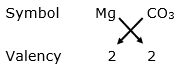
Formula: MgCO3
(ii) Cupric oxide:
Valency of cupric = 2
Valency of oxide = 2
By applying criss-cross method:

Formula: CuO
(iii) Ferric oxide:
Valency of ferric = 3
Valency of oxide = 2
By applying criss-cross method:

Formula: Fe2O3
(iv) Ammonium hydroxide
Valency of ammonium = 1
Valency of hydroxide = 1
By applying criss-cross method:
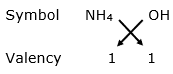
Formula: NH4OH
(v) Calcium carbonate
Valency of calcium = 2
Valency of carbonate = 2
By applying criss-cross method:
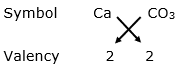
Formula: CaCO3
(vi) Potassium carbonate
Valency of potassium = 1
Valency of carbonate = 2
By applying criss-cross method:
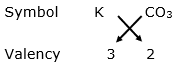
Formula: K2CO3