Describe
Thomson’s model of an atom.
Thomson’s model of an atom: -
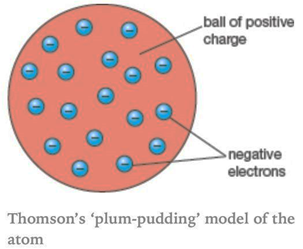
Thomson proposed the model of an atom to be similar to that of a Christmas pudding. The electrons in a sphere of positive charge, where like currants (dry fruits) in a spherical Christmas pudding.
Thomson proposed that:-
i. An atom consists of a sphere of positive charge with negatively charged electrons embedded in it.
ii. The positive and the negative charges in an atom are equal in magnitude due to which an atom as a whole is electrically neutral.
The diagram is shown below:
15