Which of the following in Fig. do not represent Bohr’s model of an atom correctly?
(i) 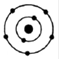
(ii) 
(iii) 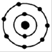
(iv) 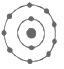
The maximum number of electrons that can be accommodated in the first shell is 2 and that in the 2nd shell is 8.
In fig ii, the number of electrons in the first shell is 4 and in fig iv the number of electrons in the second shell is 9 both of which are above the allowed capacity. So, they do not represent Bohr’s model of an atom correctly.
Hence, the correct option is C.
7