Why alloy is considered as a mixture?
Alloy consists of metals mixed in a particular proportion. They exhibit the properties of the constituents. No new chemical properties are shown once the metals are mixed. Hence an alloy is considered as a mixture.
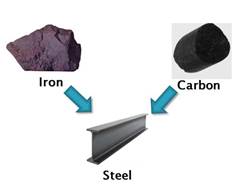
Example, steel is an alloy of iron and carbon as it is a mixture of these two elements where the larger proportion is of metal (iron) and smaller proportion of non-metal (carbon)
5