How will you separate a mixture containing benzene and diesel (difference in their boiling points is more than 50°C), which are miscible with each other?
A mixture of 2 miscible liquids whose boiling points differ by more than 50°C can be separated using a technique called distillation. The liquids must not decompose upon boiling.
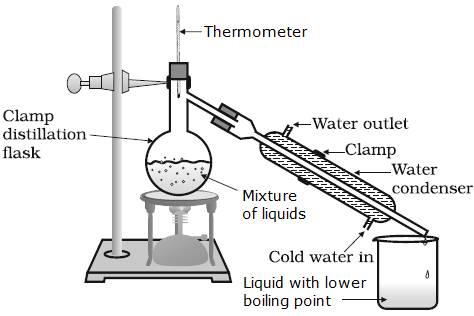
The mixture of liquids is taken in a round bottom flask and is heated. The liquid component with a lower boiling point starts to escape from the mixture and the gas is passed through a condenser. The cold water helps to condense the gas into its liquid form and is collected in a beaker.
In the given mixture, at around 80°C, benzene gets converted into a gaseous state and is sent through the condenser to get benzene back in the liquid state.
Finally, diesel remains in the round bottom flask and benzene in the beaker.