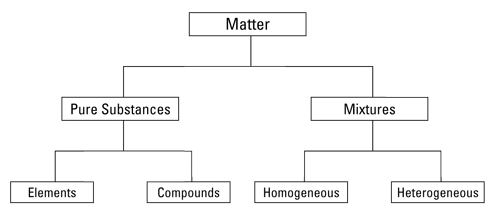
Give two characteristics each of:
(a) Pure substances.
(b) Mixtures.
(a) A pure substance is a type of matter which is formed by a single type of particles. The constituent particles are identical in their chemical nature. The constituent particles of a particular pure substance will always be found in a fixed composition and cannot be separated using physical methods.
Elements and compounds are examples of pure substances.
(b) Mixtures are a type of matter formed by more than one pure substance. The constituent particles just mix together in different proportions to give different mixtures. The properties exhibited by the mixture are the same as the properties exhibited by the individual constituent particles. These constituents can be fairly separated using physical techniques.
Sugar solution, milk, air are examples of mixtures.
Note: The sub-division of matter is shown as: