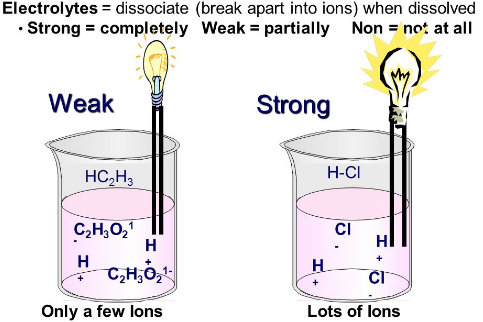
An aqueous solution of an acid conducts electricity. Give reason.
Any solution will conduct electricity only when it has free mobile ions in it. So in the case of the aqueous solution of acids, free ions of hydrogen are present in the solution which makes the solution to behave like an electrolyte and hence conducts electricity. Depending upon the number of free hydrogen ions, acidic electrolytes can be further classified as a strong and weak electrolyte.
6