What are the products formed when an acid reacts with a base? What is the type of reaction? Give one example and name the salt obtained.
The reaction of an acid with a base is a common reaction. It is a double decomposition type of reaction in which exchange of cations and anions takes place. The acid reacts with the base to form salt and water and the reaction is termed as neutralization reaction.
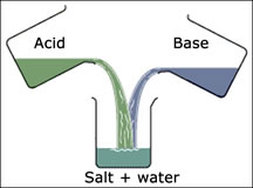
Let us take the example of sodium hydroxide reacting with hydrogen chloride. The reaction is as follows:
NaOH + HCl → NaCl + H2O
So the salt obtained is called as sodium chloride.
12