A gas X reacts with lime water and forms compound Y which is used as a bleaching agent in chemical industry. Identify X and Y. Give the chemical equation of the reaction involved.
The most commonly used bleaching agent in the industry is bleaching powder or calcium oxychloride.
It is prepared by passing the chlorine gas through dry slaked lime. The reaction is given as follows:
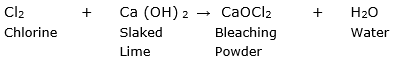
So X is chlorine and Y is calcium oxychloride.
14