How will you prove that a given salt is a carbonate of a metal?
To prove that the given salt is a carbonate of a metal, add a few drops of Hydrochloric or Sulphuric acid to the salt and when the reaction is completed brisk effervescence is observed and pass the escaping gas through lime water. Lime water will turn milky in appearance which proves that the liberated gas is carbon dioxide and the salt was a carbonate of a metal.
The above explanation can be described by following reactions:
MCO3 + HCl → MCl + H2O + CO2
Where M is any metal
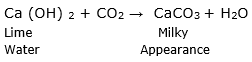
17