For each of the compound A and B, suggest a suitable method its preparation along with the balanced chemical equations.
i. A is bleaching powder
ii. B is gypsum
(a) (i) The most commonly used bleaching agent in the industry is bleaching powder or calcium oxychloride.
It is prepared by passing the chlorine gas through dry slaked lime. The reaction is given as follows:
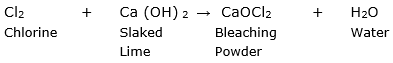
(ii) Gypsum is a naturally occurring mineral form of hydrated calcium sulphate. It can be obtained from Plaster of Paris by adding water to it. The reaction is as follows:
CaSO4.1/2H2O + 3/2 H2O → CaSO4.2H2O
18