With the help of a labelled diagram, explain the process of electrolytic refining of copper.
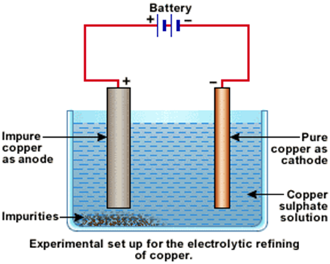
The process of electrolytic refining of copper:
i. In this electrolytic refining, the electrolyte is a solution of copper sulphate.
ii. In this process, the anode is impure copper, whereas the cathode is a thin strip of pure copper.
iii. On passing the current through the electrolyte, the pure copper from the anode dissolves into the electrolyte.
iv. An equivalent amount of pure copper from the electrolyte is deposited at the cathode.
v. The insoluble impurities settle down at the bottom of the anode (the positively charged electrode) are known as anode mud.
18