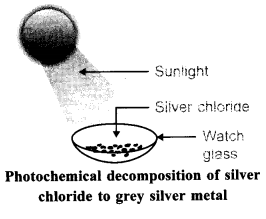
What change will you observe if white silver chloride is placed in sunlight? Write an equation for the reaction and the type of the reaction.
Most of the compounds of silver are sensitive to light and hence silver compounds are stored in dark coloured bottles. Similarly silver chloride is also a light sensitive compound that is it gets decomposed when exposed to light.
So when silver chloride is exposed to sunlight, it gets decomposed to give silver metal and chlorine gas is liberated. The reaction is called as photolysis reaction. The word photolysis can be broken into two parts photo means light and lysis means breaking. So photolysis means breaking of a compound in the presence of light.
The reaction is given as follows:
2AgCl → 2Ag + Cl2
8