Identify the type of chemical reaction taking place
i. on making a solution of potassium chloride with silver nitrate, an insoluble white substance is formed.
ii. on heating green coloured ferrous sulphate crystals, reddish-brown solid is left and smell of a gas having odor of burning Sulphur is observed.
(i) The reaction is as follows:
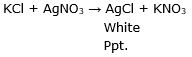
When potassium chloride reacts with silver nitrate, a white precipitate of silver chloride is formed.
This reaction is a type of Precipitation reaction.
(ii) The reaction is as follows:
2FeSO4→ Fe2O3 + SO2 + SO3
In the above reaction, ferrous sulphate decomposes to give reddish brown solids of iron oxide and the gas liberated is sulphur dioxide and sulphur trioxide which gives an odor of burning sulphur.
11