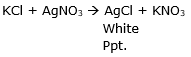Identify the type of each of the following reactions:
a. A reaction in which a single product is formed from two or more reactants.
b. The reaction mixture becomes warm.
c. An insoluble substance is formed.
(a) A reaction in which is a single product is formed from two or more reactants is a combination reaction. The example of combination reaction is as follows:
H2 + O2→ H2O
(b) Exothermic reaction is a reaction in which heat generated during the reaction process. So the reaction mixture becomes warm is a type of exothermic reaction. So an example of an exothermic reaction is as follows:
N2 + 3H2→ 2NH3 + Heat
(c) Precipitation reaction is the reaction in which an insoluble mass is formed at the end of the reaction. The example of such reaction is as follows: