You are provided with two containers made up of copper and aluminum. You are also provided with solutions of dilute HCl, dilute HNO3, ZnCl2, and H2O. In which of the above containers these solutions can be kept?
The above-mentioned solutions can be kept safely in a copper container instead of an aluminium container.
Copper is a less reactive metal and is placed below hydrogen and aluminum in the activity series and hence copper would not react with the solutions.
On the other hand aluminum is more reactive metal and it will react with the solutions to form corresponding compounds.
The activity series of metals is as follows:
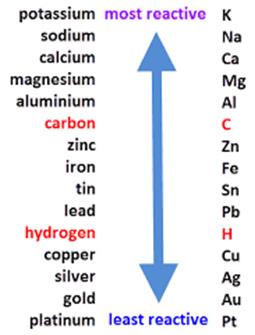
21