(i) Define corrosion. Under what conditions does corrosion take place?
(ii) Give the formula and the chemical name of rust.
(i) Corrosion is a natural process of wasting of metal due to reaction of the metals with environmental factors like moisture, oxygen etc to form corresponding metallic compounds. The metal undergoing corrosion losses its strength, appearance and its efficiency.
Corrosion generally takes place under following conditions:
1. Exposure of metals to humid climatic conditions for a long period of time
2. Exposure of metals to reactive gases and liquids for long period of time.
3. Environmental condition of the place where metal is placed like temperature of the surrounding, moisture content in the air, etc.
(ii) The chemical formula for rust is Fe2O3.nH2O(s).
Where n is the water of crystallization.
The rust is formed by the following equation:
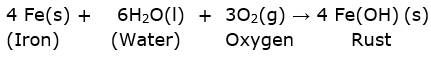
The iron hydroxide formed dehydrates to produce Fe2O3.nH2O(s).