Two carbon compounds X and Y have the molecular formula C3H6 and C4H10 respectively. Which one of the two is most likely to show addition reaction? Justify your answer. Also give the chemical equation to explain the process of addition reaction in this case.
The addition reaction is only exhibited by the unsaturated hydrocarbons because this reaction involves the breaking of multiple bonds presents between atoms to form saturated hydrocarbons.
Here, C3H6 is an alkene which is unsaturated and C4H10 is an alkane, which is saturated. Hence, C3H6 is more likely to undergo an addition reaction.
In addition reaction, the reactant breaks the multiple bonds present between atoms of the unsaturated compound. Then it later occupies the compound by forming a single bond between carbon and the reacting species.
e.g. 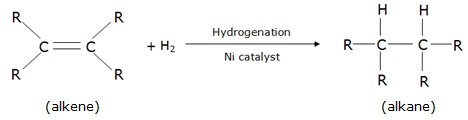
As one can see, hydrogen (reacting species) breaks the double bond between the carbon atoms and forms single bonds with them resulting in an alkane.