A compound X is formed by the reaction of a carboxylic acid C2H4O2 and an alcohol in presence of a few drops of H2SO4. The alcohol on oxidation with alkaline KMnO4 followed by acidification gives the same carboxylic acid used in this reaction. Give the names and structures of the
a. carboxylic acid,
b. alcohol and
c. compound X.
Also, write the reaction.
Given that,
C2H4O2 (Acid) + Alcohol → X (in the presence of H2SO4)
The above reaction is esterification reaction.
Given that the alcohol upon oxidation with alkaline KMnO4 gives the carboxylic acid C2H4O2. This implies that the alcohol contains 2 carbons as well.
Therefore, the alcohol is ethanol and the carboxylic acid is ethanoic acid.
The condensed structural formula of alcohol used:
CH3—CH2—OH
The condensed structural formula of carboxylic acid used:
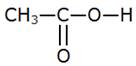
The compound X is then ethyl ethanoate,
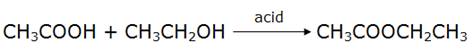
where CH3COOCH2CH3 is X.