What are hydrocarbons? Write the general formula of (i) saturated hydrocarbons, and (ii) unsaturated hydrocarbons and draw the structure of one hydrocarbon of each type.
Hydrocarbons are compounds which contain carbon and hydrogen only. The elements (carbon and hydrogen) form covalent bonds between them.
(i) Saturated hydrocarbons are represented by the molecular formula CnH2n+2, where ‘n’ is the number of carbon atoms. Only single bonds exist between the atoms in saturated hydrocarbons.
An example of a saturated hydrocarbon is methane (CH4). Carbon can form a maximum of 4 bonds and all 4 are satisfied by hydrogen atoms.
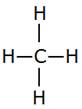
Methane
(ii) Unsaturated hydrocarbons are hydrocarbons containing at least one double/triple bond. If the hydrocarbon is an alkene, then the general molecular formula is CnH2n, and if the hydrocarbon is an alkyne, then the molecular formula is CnH2n-2.
An example of alkene is propene (C3H6) in which n=3. One of the carbon-carbon bonds is a double bond. The other carbon-carbon bond is a single bond. The remaining valency of each carbon is satisfied by hydrogen atoms.
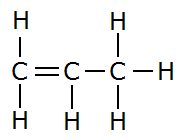
An example of an alkyne is ethyne (C2H2) in which n=2. Carbon can form 4 bonds with neighbouring atoms. Here, the carbon-carbon bond is a triple bond. So, the remaining 1 bond of each carbon is formed with a hydrogen atom.
H—C≡C—H