An organic compound ‘A’ on heating with another compound ‘B’ in presence of concentrated sulphuric acid forms a sweet smelling compound ‘C’:
i. Identify the name of this chemical reaction.
ii. Write a balanced chemical equation for the above chemical reaction.
iii. Write one use of compound ‘C’.
iv. Write a balanced chemical equation for the reaction when an acid or a base is added to compound ‘C’.
The reaction mentioned in the question is:
![]()
(i) Given that C is sweet smelling. Hence, C is an ester. Therefore the reaction mentioned is esterification reaction, where the reactants are alcohol and carboxylic acid.
i.e., A – alcohol, B – carboxylic acid (or vice versa)
(ii) R-OH represents an alcohol and R’-COOH represents a carboxylic acid, where R, R’ are different alkyl groups (such as methyl group (-CH3), butyl group (C4H9) etc). Then, esterification reaction is given by:
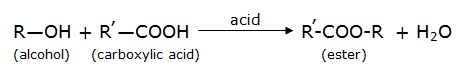
(iii) Since esters are sweet smelling substances, they are used in making perfumes and as flavouring agents in foods.
(iv) Esters upon hydrolysis (water is one of the reactants) in the presence of an acid or base gives back the alcohol and the carboxylic acid from which the ester was formed. This reaction is called saponification reaction.
![]()