Match the reactions given in Column (A) with the names given in Column (B).
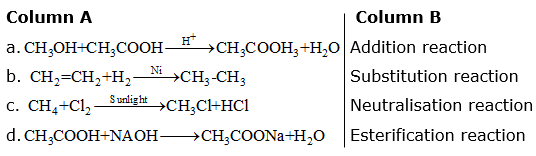
(a) – Esterification reaction
(b) – Addition reaction
(c) – Substitution reaction
(d) – Neutralisation reaction
(a) Here, an alcohol (CH3OH) reacts with a carboxylic acid (CH3COOH) reacts in the presence of an acid to form an ester (CH3COOCH3). This reaction is called esterification reaction.
(b) The alkene is converted to alkane by the addition of hydrogen. The reaction is called an addition reaction.
(c) One of the hydrogen atoms is replaced by chlorine in the presence of sunlight and hence the reaction is called substitution reaction.
(d) Here, an acid (CH3COOH) reacts with a base (NaOH) to produce salt and water. This reaction is called a neutralisation reaction.
31