What is meant by “structural isomers”? Give reason why propane (C3H8) cannot exhibit this characteristic. Draw the structures of possible isomers of butane (C4H10).
Structural isomers are those compounds which have the same molecular formula but different structural formula. The basic structure or skeleton of the carbon chain would be different in each of these compounds.
The minimum number of carbons required to form at least one branch is 4 (3 carbons in parent chain and 1 carbon in side chain). The possible skeletons using 4 carbon atoms are:
C—C—C—C
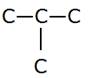
Using 3 carbons, one can only produce the following skeleton:
C—C—C
Hence, propane (containing 3 carbon atoms) cannot exhibit structural isomerism.
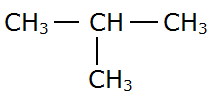
The possible structural isomers of butane (C4H10) are:
(a) n-butane:
![]()
(b) 2-methylpropane: