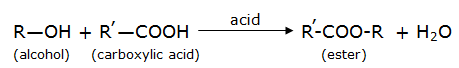Esters are sweet-smelling substances and are used in making perfumes. Suggest some activity and the reaction involved for the preparation of an ester with well labeled diagram.
Preparation of ester:
Take a small quantity (for example, 1 ml) of ethanol (or any alcohol in general) and a small quantity of acetic acid (or any carboxylic acid) in a test tube. Add a few drops of concentrated sulphuric acid to the mixture.
Heat the test tube in a water bath for about five minutes as shown in the figure.
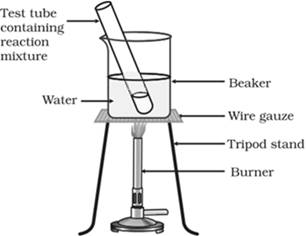
Then, pour the contents of the test tube into a beaker containing 20-50 ml of water. Smell the mixture in the beaker. One could notice a sweet fruity smell coming from the beaker. This smell is produced due to the formation of ester from alcohol and carboxylic acid. The reaction is called esterification reaction.
The reaction can be represented as: