Give reasons why sodium and potassium are stored in kerosene?
Sodium and potassium are highly reactive metals. Let us look at the activity series of the metal.
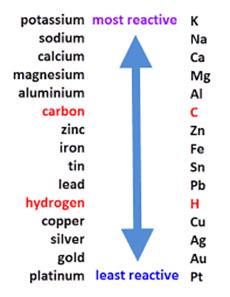
• When potassium and sodium are kept in open air, they react very violently with the atmospheric oxygen and this reaction is so vigorous that it can catch fire easily and a lot of heat if produced during the reaction.
• On the other hand, kerosene provides an inert medium as sodium and potassium are unreactive in kerosene oil. Kerosene oil has low density and these two metals sink in the oil hence minimizing the chances of reactions with surroundings.
So, to prevent the violent reaction, potassium and sodium is kept immersed in kerosene oil.


1