The position of some elements A,B,C,D,E,F and G in the Modern Periodic Table is given as under:
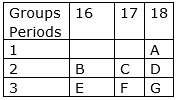
a. In which group are inert elements placed?
b. What type of ions would B, C, E and F form?
c. Which elements would have chemical properties similar to C?
d. How many shells would A have?
e. What is the similarity between A and D?
f. Identify the most abundant element in the earth’s crust.
(a) Inert elements are placed in group 18.
(b) Elements B and E are placed in group 16 that means these have 6 electrons in outermost shell and these elements require 2 electrons to acquire noble gas configuration. So elements B and E will form B2- and E2- type ions.
And elements C and F are placed in group 17 that means these have 7 electrons in outermost shell and these elements require only 1 electron to acquire noble gas configuration. So elements C and F will form C- and F- type ions.
(c) We know that elements with similar properties are placed in same group. So element F would have chemical properties similar to C.
(d) Since A is placed in 1st period so it has only one shell.
(e) A and D are placed in group 18 so these both elements have 8 electrons in outermost shell.
(f) Most abundant element in the earth’s crust is B because B is oxygen and oxygen is most abundant element in earth’s crust.