(i) Distinguish between ionic and covalent compounds under the following properties:
(a) The strength of forces between constituent elements
(b) The solubility of compounds in water
(c) Electrical conduction in substances
(ii) Explain how the following metals are obtained from their compounds by the reduction process:
(a) Metal M which is in the middle of the reactivity series.
(b) Metal N which is high up in the reactivity series.
Given one example of each type.
OR
(i) Distinguish between ‘roasting’ and ‘calcination’. Which of these two is used for sulphide ores and why?
(ii) Write a chemical equation to illustrate the use of aluminium for joining cracked railway lines.
(iii) Name the anode, the cathode and the electrolyte used in the electrolytic refining of impure copper.
(i) IONIC COMPOUND: (a) Formed due to the electrostatic force of attraction that is, electrovalent bond.
(b) Soluble in polar solvents like water as it readily forms ions but not soluble in non-polar solvents like benzene.
(c) Conduct electricity only in a molten state as it forms ions.
COVALENT COMPOUND: (a) Has covalent bond between constituents due to polarity.
(b) Soluble in non-polar solvents like benzene as it readily forms ions but not soluble in polar solvents like water.
(c) Does not conduct electricity.
(ii) There are two main methods of extracting metals from their ores:
* reduction with carbon
* electrolysis
The method used to extract a metal from their compounds depends upon the stability of its compound in the ore. And, the stability of the ore depends on upon the reactivity of the metal. The following diagram shows the brief methods for extraction.
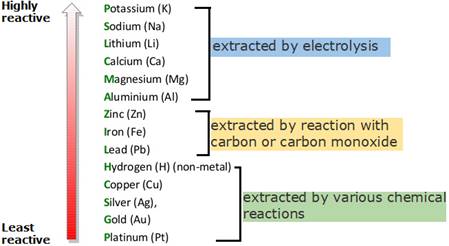
(a) Metal M which is in the middle of the reactivity series –
The metals less reactive than carbon can be extracted from their ores by reduction.
For example, the extraction of lead from lead oxide:![]()
(b) Metal N which is high up in the reactivity series –
Metals above carbon in the reactivity series must be extracted using electrolysis.
For example, when an electric current is passed through molten state or solution of sodium chloride, sodium metal deposited over the cathode.
Na+ + e− ⇒ Na
2Cl− − e− ⇒ Cl2
2NaCl ⇒ 2Na + Cl2
Metals obtained from the process of electrolytic reduction are pure in form.
OR
(i) Roasting: When sulphide containing ore is heated for a long time in the presence of an excess of air to form a metal oxide, this method of converting sulphide containing ore into metal oxide in the presence of an excess of air is called roasting.
e.g.,![]()
Calcination: When carbonate containing ore is heated for a long time in the absence of air it is converted into metal oxide. This method of converting carbonate containing ore into metal oxide is called calcination.
e.g.,![]()
(ii)Write a chemical equation to illustrate the use of aluminum for joining cracked railway lines.
Thermite reaction used for the welding of rails (joining metals) called as thermite welding.
This is an exothermic reaction which includes the igniting aluminum and ferric oxide.
Fe2O3 (s) + Al (s) →Al2O3 + 2 Fe(l) + Heat
Iron obtained in this process is in a molten state.
(iii) Name the anode, the cathode and the electrolyte used in the electrolytic refining of impure copper.
In the method of electrolysis, the rod of impure metal is taken as anode and rod of pure metal is taken as a cathode. The aqueous solution of a salt of, metal is used as an electrolyte. On passing electric current the anode dissolves in the electrolyte, and the same proportion of pure metal gets deposited on the cathode.
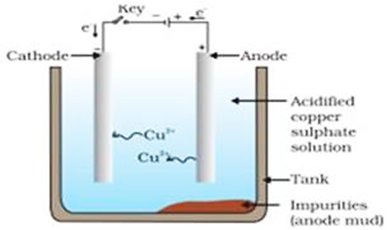
Refining of copper by electrolysis