A salt X is formed, and gas is evolved when ethanoic acid reacts with sodium hydrogen carbonate.
Name the salt X and the gas evolved. Describe an activity and draw the diagram of the apparatus to prove that the evolved gas is the one which you have named. Also, write chemical equation of the reaction involved.
Salt X is sodium ethanoate and the evolved gas is carbon dioxide.
The reaction is given below:
CH3COOH + NaHCO3 → CH3COONa + H2CO3
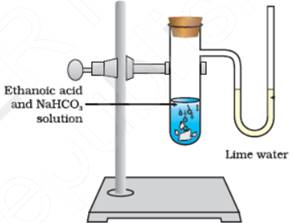
Activity:
(a) Take a test tube and a bent tube.
(b) Take ethanoic acid and sodium bicarbonate solution in the test tube.
(c) Insert the bent tube in the cork and fit the cork at the mouth of the test tube.
(d) Fill lime water in the bent tube so that lime water is in the ‘U’ portion of this tube.
After some time; it is observed that the lime water turns milky. This confirms that the evolved gas is carbon dioxide. The diagram is given below: