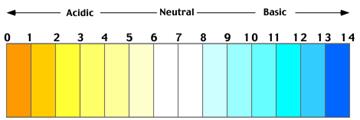
The pH values of four solutions A, B, C and D as determined by a student 3, 7, 12, and 8 respectively
i) Identify the most acidic and most basic solutions.
ii) Arrange the above four solutions in the decreasing order of their hydrogen ion concentration.
Less is the pH of a solution; more the solution is acidic in nature.
More is the pH of a solution; more the solution is basic in nature.
i) Among the solutions:
A = 3
B = 7
C = 12
D = 8
A is more acidic because its pH value is very less. On the other hand, C is more basic because its pH value is high.
ii) As the acidic nature increases, the concentration of hydrogen ions also increases. As the basic nature increases, the concentration of hydrogen ions decreases.
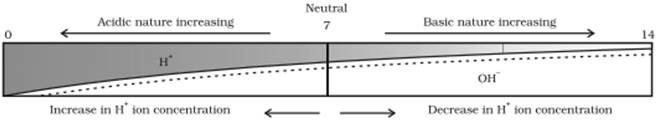
Hence the order of the given solutions in the decreasing order of their hydrogen ion concentration is given as:
A < B < D < C