An element X (atomic number 17) reacts with an element Y (atomic number 20) to form a divalent halide.
i. Where in the Periodic Table are elements X and Y placed?
ii. Classify X and Y as metal(s),non-metal(s) or metalloid (s).
iii. What will be the nature of oxide of element Y? Identify the nature of bonding in the compound formed.
iv. Draw the electron dot structure of the divalent halide.
The following table shows the position of six elements A, B, C, D, E and F in the Periodic Table.
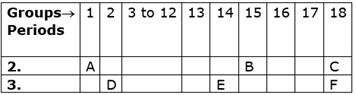
Using the above the table answer the following questions:
i. Which element will from only covalent compounds?
ii. Which element is a metal with valency 3?
iii. Which element is a non-metal valency 3?
iv. Write a common name for the family of elements C and F.
v. Out of D and E, which one has a bigger atomic radius and why?
i. X is placed in the third period and 17th group. (This is due to the electronic configuration of the element which is 2,8,7)
Y is placed in the third period and 2nd group (This is due to its electronic configuration-2,8,8,2)
Important: The number of shells gives the period in which the element is placed.
The outer electronic configuration of the elements determines the group in which it is placed. Elements with 7 valence electrons will fall into the 17th group and 2 valence electrons will fall into the 2nd group).
ii. X is a non-metal and Y is a metal.
Important: X is a non-metal as it is present in the right-hand side of the periodic table (metallic character is the lowest here). Similarly, Y is a metal as it is present on the left-hand side of the periodic table. (metallic character is the highest here).
iii. The oxide of Y will be acidic. The nature of bonding will be ionic.
(Reason: Oxides of metals are acidic and ionic in nature and oxides of non-metals are basic and covalent in nature)
iv. 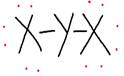
The divalent halide formed is YX2.
i. B forms only covalent compounds. (Reason: B has a very small size)
ii. Doesn’t exist
iii. B is a non-metal with valency 3 (reason: It has seven valence electrons)
iv. Noble gases (Reason: They are inert as they are very stable)
v. D has a bigger atomic radius as atomic radius decreases across a period.