Atoms of eight elements A, B, C, D, E, F, G and H have the same number of electronic shells but different number of electrons in their outermost shell. It was found that elements A and G combine to form an ionic compound. This compound is added in a small amount to almost all vegetable dishes during cooking. Oxides of elements A and B are basic in nature while those of E and F are acidic. The oxide of D is almost neutral. Based on the above information answer the following questions:
i. To which group or period of the periodic table do the listed elements belong?
ii. What would be the nature of compound formed by a combination of elements B and F?
iii. Which two of these elements could definitely be metals?
iv. Which one of the eight elements is most likely to be found in gaseous state at room temperature?
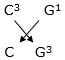
v. If the number of electrons in the outermost shell of elements C and G be 3 and 7 respectively, write the formula of the compound formed by the combination of C and G.
i. and B belong to group 1 and 2 because they form basic oxides. C belongs to group 13 as it has 3 valence electrons. D belongs to group 14 as it forms almost neutral oxide. E and F belong to group 15 and 16 as they form acidic oxides, G belongs to group 17 as it has 7 valence electrons and H belongs to group 18. They belong to 3rd period of the Periodic Table because AG is NaCI, added in a small amount to almost all vegetable dishes during cooking and Na and Cl belong to 3rd period.
ii. onic compounds will be formed because ‘B’ is metal and ‘F’ is non-metal. ‘B’ can lose two electrons and ‘F’ can gain two electrons.
Ionic compounds are compounds made up of ions. These ions are atoms that gain or lose electrons, giving them a net positive or negative charge. Metals tend to lose electrons, so they become cations and have a net positive charge. Nonmetals tend to gain electrons, forming anions that have a net negative charge.
iii. A and B are definitely metals as they form basic oxides.
Metal oxides are basic in nature because they react with dilute acids to form salt and water. They also dissolve in water to form metal hydroxides which are alkaline in nature. These metal hydroxides release OH- ions, so they are basic.
iv. Elements G and H are gaseous at room temperature
v. CG3 is the formula of the compound formed by combination of C and G.
C has the valence electrons of 3 therefore its valency will be 3
G has valence electrons as 7 therefore its valency will be 1
Formula