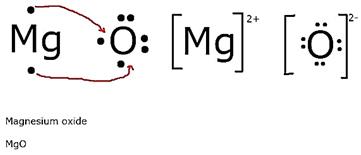An element placed in 2nd Group and 3rd Period of the Periodic Table, burns in presence of oxygen to form a basic oxide.
i. Identify the element.
ii. Write the electronic configuration.
iii. Write a balanced equation when it burns in the presence of air.
iv. Write a balanced equation when this oxide is dissolved in water.
v. Draw the electron dot structure for the formation of this oxide.
(i) Since the element is placed in 2nd group and 3rd period of the periodic table then this means the element has 2 valence electrons and 3 shells. So it is Magnesium (Mg).
(ii) Its electronic configuration is 2, 8, 2.
(iii) The chemical equation when it burns in the presence of air is
2Mg + O2→ 2MgO
(iv) The balanced equation when this oxide is dissolved in water is
MgO + H2O → Mg(OH)2
(v)