What happens to the ph of the solution when we increase the temperature of the solution? Explain?
On heating, a solution the pH of the medium get shifts to the right side in the scale in a temperature increase because of the ability of a solution to ionizer increases and so the concentration of H+ ions increases in the solution.
The pH scale is given below: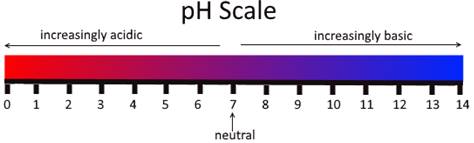
14