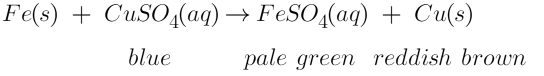AIM
(i) To observe the action of Zn, Fe, Cu and Al metals on the following salt solutions :
(a) ZnSO4(aq.)
(b) FeSO4(aq.)
(c) CuSO4(aq.)
(d) Al2(SO4)3(aq.)
(ii) To arrange Zn, Fe, Cu and Al (metals) in the decreasing order of reactivity based on the above result.
MATERIALS REQUIRED
Apparatus: Test tubes, test stand,
Chemical Compounds: Metals-Zn granules, Fe filings, Cu turnings, Al foil and aqueous solutions of zinc sulphate, ferrous sulphate, copper sulphate, and aluminium sulphate.
THEORY
Metals are elements which are good conductors of heat and electricity. Most metals lose electrons in chemical reactions to become electropositive. The alkali metal comprises group 1 of the periodic table.
1. The metals have been arranged in the decreasing order of reactivity in a series. This series is known as the reactivity or activity series.
2. The reactivity series of metals is used for predicting the products of displacement reactions and the reactivity of metals in other reactions. Potassium is the most reactive metal, while platinum is the least reactive.
3. A more reactive metal can displace less reactive metals from its salt solution or compounds If metal C displaces metal B from its salt solution, but metal A displaces metal C from its salt solution it follows that the increasing order of reactivity is :
B < C < A.
4. Displacement reactions are prevalent in metals due to the relative strength of metal to displace other metals from their salt solution. In a displacement reaction, a more reactive metal can displace a less reactive metal from its salt solution. The reaction is often known as metal displacement reaction.
When zinc granules are immersed in the copper sulphate solution, the initial colour of copper sulphate from blue starts get changed to colourless with reddish brown particles of pure copper settle at the bottom of the test tube. This chemical change for the following reaction :

Some other displacement reactions using zinc and iron are as follows :
![]()
![]()
Colour of solutions and metals
Name |
Formula |
Colour |
Aluminium Iron Copper Zinc Aluminium sulphate Ferrous sulphate Zinc sulphate Copper sulphate |
Al Fe Cu Zn Al2(SO4)3 FeSO4 ZnSO4 CuSO4 |
White Blackish grey Reddish brown Silvery white (greyish) Colourless Pale green Colourless Blue |
PROCEDURE
1. Take four clean, dry test tubes and label them as 'A', 'B', 'C' and 'D'.
2. Pour 10 ml of the given aqueous solution into their respective test tubes.
3. Add a small piece of Aluminum foil to each of these test tubes.
4. Observe the changes and record your observations.
5. Repeat the above steps using :
(a) Copper turnings with ZnSO4, FeSO4, CuSO4, and Al2(SO4)3 solutions.
(b) Iron filings with ZnSO4, FeSO4, CuSO4, and Al2(SO4)3 solutions.
(c) Zinc granules with ZnSO4, FeSO4, CuSO4, and Al2(SO4)3 solutions.
6. Record your observations in the following table :
OBSERVATIONS :
1. When aluminium foil (Al) is added to all the 4 test tubes:
Salt solution |
Observation |
Inference |
Al2(SO4)3 |
No change observed |
Metal cannot displace itself from its salt solution. |
FeSO4 |
The pale green colour of the solution disappears, and it becomes colourless. |
Al displaces iron from the salt solution. Therefore, Al is more reactive than Fe. |
ZnSO4 |
No change in colour of the solution, but a new coating is observed on Al.
|
Al displaces Zn from the salt solution. Therefore Al is more reactive than Zn. |
CuSO4 |
The initial colour of the solution, i.e., blue disappears, and it changes to colourless. Reddish brown deposits are observed on Al.
|
Al displaces Cu from the salt solution. Therefore Al is more reactive than Cu. |
2. When copper turnings (Cu) are added to all the 4 test tubes:
Salt solution |
Observation |
Inference |
Al2(SO4)3 |
No change observed Cu(s) + Al2(SO4)3(aq) →No Reaction |
Cu cannot displace Al from the salt solution. Therefore, Cu is less reactive than Al. |
FeSO4 |
No change observed Cu(s) + FeSO4(aq)→ No Reaction |
Cu cannot displace Fe from the salt solution. Therefore, Cu is less reactive than Fe. |
ZnSO4 |
No change observed Cu(s) + ZnSO4(aq)→ No Reaction |
Cu cannot displace Zn from the salt solution. Therefore, Cu is less reactive than Zn. |
CuSO4 |
No change observed |
Metal cannot displace itself from its salt solution. |
3. When iron fillings are added in all the 4 test tubes:
Salt solution |
Observation |
Inference |
Al2(SO4)3 |
No change observed Fe(s) + Al2(SO4)3(aq) → No Reaction. |
Fe cannot displace Al from the salt solution. Therefore, Fe is less reactive than Al. |
FeSO4 |
No change observed |
Metal cannot displace itself from its salt solution. |
ZnSO4 |
No change observed Fe(s) + ZnSO4(aq) → No Reaction |
Fe cannot displace Zn from the salt solution. Therefore, Fe is less reactive than Zn. |
CuSO4 |
Blue solution changes to pale green, reddish brown Cu gets deposited on Fe filings.
|
Fe displaces Cu from the salt solution. Therefore, Fe is more reactive than Cu. |
4. When Zinc granules are added in all the 4 test tubes:
Salt solution |
Observation |
Inference |
Al2(SO4)3 |
No change observed Zn(s) + Al2(SO4)3(aq)→ No Reaction |
Zn cannot displace Al from the salt solution. Therefore, Zn is less reactive than Al. |
FeSO4 |
The pale green solution becomes colourless, Fe gets deposited on Zn granules.
|
Zn displaces Fe from the salt solution. Therefore, Zn is more reactive than Fe. |
ZnSO4 |
No change observed |
Metal cannot displace itself from its salt solution. |
CuSO4 |
The blue solution becomes colourless; reddish brown Cu gets deposited on Zn granules.
|
Zn displaces Cu from the salt solution. Therefore, Zn is more reactive than Cu. |
RESULT
1. Aluminium can displace Fe, Cu, and Zn from their salt solutions. Therefore Aluminum is most reactive among them.
2. Copper is unable to displace any metal among Al, Fe, and Zn from their salt solutions. Therefore Cu is the least reactive element among them.
3. Iron being less reactive than zinc is unable to displace Zn from its salt solution, whereas Zn can displace Fe from its salt solution. Therefore Zn is more reactive than Fe.
4. The decreasing order of reactivity for metals are as follows :
Al > Zn > Fe > Cu.
PRECAUTIONS
1. Handle the chemicals with care.
2. Clean each metal using sandpaper to remove the oxide layer.
3. Observe the changes patiently as few reactions may occur slowly.
4. Never taste the chemicals.
5. Rinse the test tube properly with water before use.