Draw a graph showing the variation of binding energy per nucleon versus the mass number A. Explain with the help of this graph, the release of energy in the process of nuclear fission and fusion.
Binding Energy of a nucleus is the amount of energy that needs to be supplied in order to break the nucleus into its constituents, i.e. Protons and neutrons which together constitute the nucleus and are also termed as nucleons. So more is the binding energy, more energy is required to break the nucleus, Binding energy is generally denoted by EB
However, the measure of the stability of the nucleus is generally measured in terms of Binding Energy per nucleon which is the average binding energy per nucleon, i.e. Binding energy of nucleus divided by the total number of nucleons present in the nucleus
Ebn = EB/A
Where A is the mass number of nucleus depicting the total number of nucleons of the atom, Ebn is the binding energy per nucleon
The Curve Showing Variation in Binding Energy per nucleon of elements with a mass number (A) is as shown the figure
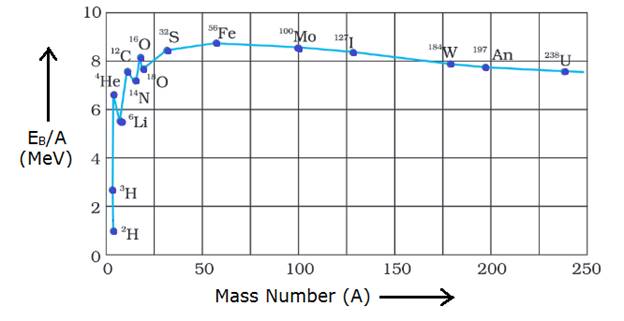
So in the curve we can see the binding energy per nucleon, is practically constant, i.e. practically independent of the atomic number for nuclei of middle mass number (30 < A < 170). Moreover, the curve has a maximum of about 8.75 MeV for A = 56 (Fe) and has a value of 7.6 MeV for A = 238 (U)
Binding Energy per Nucleon is lower for both light nuclei (A<30) and heavy nuclei (A>170)
A nuclear reaction takes place in order to decrease the energy of nuclei, i.e. increase stability hence Binding energy per nucleon, so energy is released in the process as the energy of a nucleus is being lowered, though mass number (A) or a number of nucleons is always conserved in a nuclear reaction.
Note:- Less is the energy of nucleus more is Binding energy and more is stability so if binding energy per nucleon is increased in a reaction that means more stability has been obtained and energy of nucleons has been lowered hence energy is released.
In nuclear Fission A Nuclei Breaks into two or more lighter daughter nuclei and release energy so as we can see from graph, heavy nucleons like U 238 have low Binding energy per nucleon than lighter nucleons like Fe 56 so when it breaks resulting in nuclei with higher binding energy per nucleon, i.e. lowering energy of nucleus and nucleons, i.e. increasing stability hence huge amount of energy is released in the process. So nuclear Fusion takes place with elements near the end of the graph with a higher mass number and less Binding Energy per nucleon.
In nuclear Fusion Reaction Two or more nuclei combine in order to form a new Nucleus, so as we can see from the graph lighter nuclei like He 4, C 12, C 14 have very low binding energy per nucleon and when they combine to form a heavier nucleus like Fe 56 with high Binding Energy per nucleon, in the process Binding energy per nucleon is increased by great amount hence energy of new nucleus formed is significantly lower than original nuclei’s and hence stability is increased by a huge amount leading to release in tremendous amount of energy, so it occurs with nuclei’s with lower Binding energy per nucleon found at the beginning of the graph, i.e. Nucleons with less mass number and less Binding energy per nucleon.