Consider the D–T reaction (deuterium-tritium fusion)
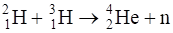
(a) Calculate the energy released in MeV in this reaction from the data:
m (21H) = 2.014102 u
m (31H) = 3.016049 u
(b) Consider the radius of both deuterium and tritium to be approximately 2.0 fm. What is the kinetic energy needed to overcome the Coulomb repulsion between the two nuclei? To what temperature must the gas be heated to initiate the reaction?
(Hint: Kinetic energy required for one fusion event = average thermal kinetic energy available with the interacting particles
= 2(3kT/2); k = Boltzmann’s constant, T = absolute temperature.)