Ionization constant of a weak base MOH, is given by the expression
Kb = 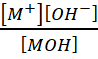
Values of ionisation constant of some weak bases at a particular temperature are given below:
Base Di-methylamine Urea Pyridine Ammonia
Kb 5.4 × 10-4 1.3 × 10-14 1.77× 10-9 1.77 × 10-5
Arrange the bases in decreasing order of the extent of their ionisation at Equilibrium. Which of the above base is the strongest?